总生存期获大幅度延长,最高超过1倍!多款肿瘤免疫治疗新药公布积极早期结果 | 一周盘点
转自:药明康德
本期看点
1. T细胞诱导剂CUE-101单药作为HPV阳性头颈部鳞状细胞癌(HNSCC)患者的二线及二线以上治疗,患者的中位总生存期(OS)较接受免疫检查点抑制剂单药治疗患者的历史报告生存期延长超过一倍。
2.肿瘤免疫治疗新药Galinpepimut-S联用帕博利珠单抗治疗Wilms肿瘤1(WT1)阳性铂耐药卵巢癌患者的早期临床数据亮眼,患者的无进展生存期(PFS)延长41%,中位OS达18.4个月。
3.选择性内皮素B受体(ETBR)抑制剂ENB-003与帕博利珠单抗联用治疗转移性铂耐药卵巢癌患者,疾病控制率(DCR)高达80%。
CUE-101:公布1期临床试验数据

CueBiopharma公司评估了其基于白细胞介素-2(IL-2)的T细胞诱导剂CUE-101作为单一疗法以及与帕博利珠单抗联用治疗复发性/转移性HPV阳性HNSCC患者的效果。
结果显示,CUE-101联用帕博利珠单抗一线治疗复发/转移性HNSCC者的客观缓解率(ORR)为47%,DCR为65%。PD-L1低表达患者的ORR为56%。接受CUE-101单药作为二线及二线以上治疗的患者,中位OS为20.8个月,而在使用免疫检查点抑制剂作为二线治疗的临床试验(CheckMate1411和KEYNOTE-040)中,患者的历史报告生存期约为8个月(分别为7.5个月和8.4个月)。安全性方面,CUE-102在剂量递增试验阶段未观察到剂量限制性毒性(DLT),也未达到最大耐受剂量。
Galinpepimut-S:公布1/2期临床试验数据

SELLASLifeSciencesGroup公司公布了其靶向WT1蛋白的潜在“first-in-class”肿瘤免疫治疗新药Galinpepimut-S(GPS)联用PD-1抑制剂帕博利珠单抗用于治疗WT1阳性铂耐药卵巢癌患者的1/2期临床试验的最终结果。该药由4条多肽链构成,抗原表位多达25个,适用于全球范围内绝大多数人类白细胞组织相容性抗原(HLA)类型,能够激发自身免疫系统对WT1抗原强烈的免疫反应。
研究结果显示,GPS联合疗法的中位OS为18.4个月。相比之下,KEYNOTE-028研究中,此类患者的中位总生存期(OS)为13.8个月,化疗标准治疗的中位OS为11-14个月。此外,GPS联合治疗产生了WT1特异性T细胞免疫反应,在亚组患者中与生物标志物IFNγ和MIP1β呈正相关。患者的PFS延长了41%,差异具有统计学意义(P=0.025)。
ENB-003:公布1b期临床试验数据

ENBTherapeutics公司公布了其在研选择性ETBR抑制剂ENB-003与帕博利珠单抗联用治疗难治性晚期实体瘤患者的1b期临床试验的顶线数据。在临床前研究中,ENB-003提高了CAR-T细胞疗法和PD-1抑制剂在多种癌症类型的实体瘤中的疗效。
此次公布的数据显示,ENB-003与帕博利珠单抗联用的耐受性良好,5例微卫星稳定(MSS)的转移性铂耐药卵巢癌患者的ORR为40%,DCR为80%。2例获得部分缓解(PR)的患者的肿瘤负荷分别减少了95%和33%。8个月的无进展生存率为60%。相比之下,PD-1抑制剂单药治疗此类患者的ORR为8%,DCR为22%,6个月的无进展生存率为20%。
MDNA11:公布1/2期临床试验的新数据
MedicennaTherapeutics公司公布了其下一代长效IL-2超级激动剂MDNA11治疗晚期实体肿瘤的新临床结果。MDNA11具有优秀的CD122(IL-2受体β)结合作用,而没有CD25(IL-2受体α)亲和力,从而能优先刺激癌症杀伤效应T细胞和NK细胞。
此次公布的结果显示,MDNA11治疗在所有队列中的耐受性良好,继续表现出持久的抗肿瘤免疫作用。MDNA11已使3名黑色素瘤患者获得了5个月至18个月的持久疾病稳定(SD),并且这些患者在免疫检查点抑制剂治疗失败后,肿瘤大小仍有缩小。1例转移性胰腺导管腺癌患者的3个病灶(2个靶病灶和1个非靶病灶)完全消褪。一例皮肤黑色素瘤患者的靶淋巴结病灶在第12周时减少了70%。这两位患者目前均为PR。
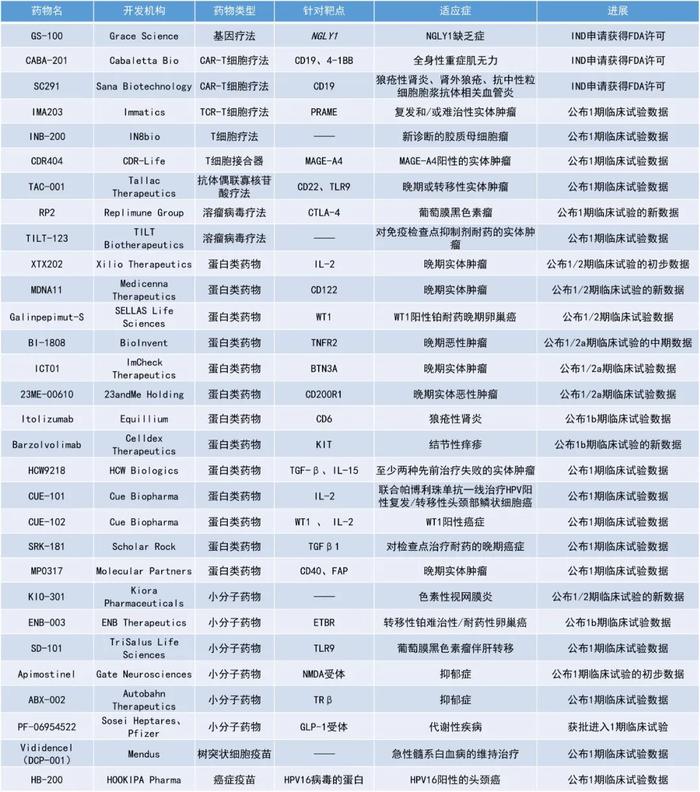
IMA203:公布1期临床试验数据
Immatics公司公布了其在研T细胞受体T细胞(TCR-T)疗法IMA203治疗复发性和/或难治性实体瘤患者的1期试验的中期数据。IMA203是由Immatics专有ACTengine平台所开发的TCR-T细胞,靶向由HLA-A*02呈递的黑素瘤抗原PRAME。PRAME是一种在各种实体瘤中经常表达的蛋白质。
此次数据公布以黑色素瘤患者为重点,IMA203GEN1TCR细胞疗法的1a期和队列A的耐受性良好,黑色素瘤患者按推荐的2期剂量接受治疗后,确认的ORR为50%;一些患者持续缓解超过15个月,中位随访时间为14.4个月时仍未达到中位缓解持续时间。
IMA203CD8是该公司的第二代产品,药理作用增强,作用模式也与IMA203有所区别。接受IMA203CD8GEN2TCR细胞疗法治疗的患者(队列C)的耐受性可控,剂量递增研究仍在进行中。在接受剂量3和剂量4治疗的患者中观察到初步的临床活动,确认的ORR分别为56%(5/9)和58%(7/12);7个获得缓解的患者中有6个还保持着缓解,最长已缓解超过12个月。
此外,研究人员在非黑色素瘤患者中也观察到了疗效信号,包括卵巢癌、子宫癌、非小细胞肺癌和三阴性乳腺癌。
SRK-181:公布1期临床试验数据
ScholarRock公司公布了其旨在克服晚期癌症患者对检查点治疗的耐药性的单克隆抗体SRK-181的1期概念验证试验数据。TGFβ1是在许多人类肿瘤类型中表达的主要TGFβ亚型。研究表明,TGFβ1是免疫抑制肿瘤微环境的关键贡献者。SRK-181是一种选择性的潜在的TGFβ1激活抑制剂,与抗PD-1/PD-L1疗法联合使用时,有潜力克服检查点治疗耐药问题,同时可能避免其他非选择性TGFβ抑制方法存在的心脏毒性。
此次公布的数据显示,SRK-181联用帕博利珠单抗治疗此前已接受过多线治疗的透明细胞肾细胞癌(ccRCC)患者具有良好的抗肿瘤活性,且患者的耐受性良好。患者的ORR为21.4%,疾病控制率为57%,生物标志物数据为多种肿瘤类型提供了机制证明。
CUE-102:公布1期临床试验数据
CueBiopharma公司公布了CUE-102的初步临床数据。CUE-102是一种由两个呈递WT1肽的人白细胞抗原(HLA)分子、四个亲和力减弱的IL-2分子和一个效应减弱的人免疫球蛋白G(IgG1)Fc结构域组成的新型生物制品,被开发作为单一疗法治疗WT1阳性复发/转移性癌症患者。WT1已知在多种实体瘤和血液系统恶性肿瘤中过度表达,包括胃癌、胶质母细胞瘤、胰腺癌、卵巢癌、子宫内膜癌、乳腺癌、肺癌、结直肠癌和急性髓系白血病等。此次公布的结果显示,CUE-102在4mg/kg和2mg/kg剂量下的DCR分别为75%和80%,其中有两名患者的肿瘤缩小了30%和29%。
Itolizumab:公布1b期临床试验数据
Equillium公司公布了其潜在“first-in-class”的CD6单克隆抗体itolizumab治疗狼疮性肾炎患者的早期临床数据。数据显示,在霉酚酸酯(MMF)和皮质类固醇的基础上加用皮下给药的itolizumab,接受28周治疗的受试者的完全缓解(CR)率为40%(6/15),PR率为33%(5/15),73%的受试者的尿蛋白肌酐比值(UPCR)下降超过50%。此外,itolizumab具有良好的安全性和耐受性。
HCW9218:公布1期临床试验数据
HCWBiologics公司公布了其皮下注射的双功能融合蛋白复合物HCW9218在至少两线治疗失败的实体瘤患者中的疗效。HCW9218创新性地将TGF-β受体和IL-15结合在一起,前者可中和肿瘤分泌的一种免疫抑制细胞因子,后者则是一种强效细胞因子,可刺激自然杀伤(NK)细胞和CD8+T细胞的细胞毒性。
此次公布的结果显示,50%(4/8)的患者实现了SD,患者的病情稳定期超过6个月。卵巢癌患者的疗效优于其他癌种的患者,SD率为66%(2/3)。HCW9218可强烈促进患者血液中NK和T细胞的增殖和活化,但不会引起细胞因子释放综合征。此外,未观察到肝酶升高。
SD-101:公布1期临床试验数据
TriSalusLifeSciences公司公布了其C类TLR9激动剂SD-101联合纳武利尤单抗治疗葡萄膜黑色素瘤伴肝转移患者的初步数据。该研究旨在评估由FDA批准的TriNav输液系统通过该公司专有的压力赋能的递送(Pressure-EnabledDrugDelivery,PEDD)方法递送SD-101是否可以改善全身性免疫检查点抑制剂治疗葡萄膜黑色素瘤伴肝转移患者的疗效。
研究结果显示,当通过PEDD方法进行给药时,SD-101联合纳武利尤单抗使大多数患者的肿瘤微环境得到调节,这与此前在针对胰腺癌患者的研究中观察到的结果一致。SD-101联用纳武利尤单抗的最佳生物制品剂量为2mg,患者的中位无进展生存期为11.7个月,DCR为81%。
大家都在看
即可访问业务对接平台,填写业务需求信息
[1]MendusPhase1vididencelclinicaltrialresultsinAMLandhigh-riskMDSpatientspublishedinpeer-reviewedmedicaljournal.RetrievedNovember7,2023,fromhttps://www.globenewswire.com/news-release/2023/11/03/2772977/0/en/Mendus-Phase-1-vididencel-clinical-trial-results-in-AML-and-high-risk-MDS-patients-published-in-peer-reviewed-medical-journal.html
[2]MolecularPartnersPresentsUpdatedPositiveDatafromOngoingPhase1TrialofMP0317(FAPXCD40)MonotherapyinPatientswithAdvancedSolidTumorsatthe2023SITCAnnualMeeting.RetrievedNovember7,2023,fromhttps://www.globenewswire.com/news-release/2023/11/03/2773319/0/en/Molecular-Partners-Presents-Updated-Positive-Data-from-Ongoing-Phase-1-Trial-of-MP0317-FAP-X-CD40-Monotherapy-in-Patients-with-Advanced-Solid-Tumors-at-the-2023-SITC-Annual-Meeting.html
[3]CueBiopharmaPresentsNewPositiveDatafromPhase1TrialsofCUE-101inHeadandNeckCancerandCUE-102inWilms’Tumor1PositiveCancersatSITC2023.RetrievedNovember7,2023,fromhttps://www.globenewswire.com/news-release/2023/11/03/2773318/0/en/Cue-Biopharma-Presents-New-Positive-Data-from-Phase-1-Trials-of-CUE-101-in-Head-and-Neck-Cancer-and-CUE-102-in-Wilms-Tumor-1-Positive-Cancers-at-SITC-2023.html
[4]ScholarRockPresentsNewDatafromPhase1DRAGONTrialShowingPromisingAnti-TumorActivityinAnti-PD-1ResistantMetastaticccRCCPatientsandSupportingSRK-181ContinuedTolerability.RetrievedNovember7,2023,fromhttps://www.businesswire.com/news/home/20231103455994/en
[5]SoseiHeptares’Partner,Pfizer,ProgressesItsGLP-1ReceptorAgonistPF-06954522intoaPhase1Trial.RetrievedNovember7,2023,fromhttps://www.globenewswire.com/news-release/2023/11/05/2773680/0/en/Sosei-Heptares-Partner-Pfizer-Progresses-Its-GLP-1-Receptor-Agonist-PF-06954522-into-a-Phase-1-Trial.html
[6]Late-BreakingPhase1LiverMetastasisDatafromTriSalusPresentedatSITC2023SupportsDevelopmentofInnovativeImmuno-oncologyApproachforLiverandPancreasIndications.RetrievedNovember7,2023,fromhttps://www.businesswire.com/news/home/20231104543355/en
[7]CabalettaBioReceivesFDAClearanceofCABA-201INDApplicationforTreatmentofGeneralizedMyastheniaGravis.RetrievedNovember7,2023,fromhttps://www.globenewswire.com/news-release/2023/11/06/2773926/0/en/Cabaletta-Bio-Receives-FDA-Clearance-of-CABA-201-IND-Application-for-Treatment-of-Generalized-Myasthenia-Gravis.html
[8]IN8bioPresentsBiologicCorrelativeDatafromtheINB-200Phase1TrialinNewlyDiagnosedGlioblastomaattheSocietyforImmunotherapyofCancer(SITC)38thAnnualMeeting.RetrievedNovember7,2023,fromhttps://www.globenewswire.com/news-release/2023/11/06/2774075/0/en/IN8bio-Presents-Biologic-Correlative-Data-from-the-INB-200-Phase-1-Trial-in-Newly-Diagnosed-Glioblastoma-at-the-Society-for-Immunotherapy-of-Cancer-SITC-38th-Annual-Meeting.html
[9]23andMeAnnouncesUpdatedSafetyandPreliminaryEfficacyDataFromthePhase1/2aStudyof23ME-00610,anInvestigationalAntibodyTargetingCD200R1.RetrievedNovember7,2023,fromhttps://www.globenewswire.com/news-release/2023/11/06/2774033/0/en/23andMe-Announces-Updated-Safety-and-Preliminary-Efficacy-Data-From-the-Phase-1-2a-Study-of-23ME-00610-an-Investigational-Antibody-Targeting-CD200R1.html
[10]EquilliumAnnouncesDatafromPhase1bEQUALISEStudyPresentedatthe2023AnnualMeetingoftheAmericanSocietyofNephrology.RetrievedNovember7,2023,fromhttps://www.businesswire.com/news/home/20231106290948/en
[11]SELLASLifeSciencesPresentsPositiveKeyImmunobiologicalandClinicalDatafromPhase1/2TrialofGalinpepimut-S(GPS)inCombinationwithKeytruda®inWT1+Platinum-ResistantAdvancedOvarianCancerattheInternationalGynecologicCancerSociety2023AnnualGlobalMeeting.RetrievedNovember7,2023,fromhttps://www.globenewswire.com/news-release/2023/11/06/2774155/0/en/SELLAS-Life-Sciences-Presents-Positive-Key-Immunobiological-and-Clinical-Data-from-Phase-1-2-Trial-of-Galinpepimut-S-GPS-in-Combination-with-Keytruda-in-WT1-Platinum-Resistant-Adva.html
[12]MedicennaAnnouncesPromisingSingle-AgentResponseandDurabilityofMDNA11inthePhase1/2ABILITYStudyDuringDoseEscalationatthe38thAnnualMeetingoftheSocietyforImmunotherapyofCancer(SITC).RetrievedNovember7,2023,fromhttps://ir.medicenna.com/news-releases/news-release-details/medicenna-announces-promising-single-agent-response-and
[13]AAOLate-Breaking:Kiora’sSmallMoleculePhotoswitchDemonstratesMeaningfulVisionImprovementsinBlindPatientswithRetinitisPigmentosa.RetrievedNovember7,2023,fromhttps://kiorapharma.reportablenews.com/pr/kiora-presents-abacus-data-at-aao
[14]TILTBiotherapeuticsAnnouncesPositiveClinicalDataonLeadAssetTILT-123atSocietyforImmunotherapyofCancer2023.RetrievedNovember7,2023,fromhttps://www.globenewswire.com/news-release/2023/11/06/2773739/0/en/TILT-Biotherapeutics-Announces-Positive-Clinical-Data-on-Lead-Asset-TILT-123-at-Society-for-Immunotherapy-of-Cancer-2023.html
[15]CelldexTherapeuticsPresentsPositiveDatafromPrurigoNodularisPhase1bStudyDemonstratingMeaningfulReductioninItchandSkinClearingwithSingleDose3.0mg/kgBarzolvolimab.RetrievedNovember7,2023,fromhttps://ir.celldex.com/news-releases/news-release-details/celldex-therapeutics-presents-positive-data-prurigo-nodularis
[16]XilioAnnouncesInitialMonotherapySafetyandAnti-TumorActivityDataforXTX202,aTumorActivated,Engineered,Beta-GammaIL-2,inLateLinePatientswithAdvancedSolidTumors.RetrievedNovember7,2023,fromhttps://ir.xiliotx.com/node/7816/pdf
[20]TallacPresentsFirstClinicalDataforTAC-001atSITC2023.RetrievedNovember7,2023,fromhttps://www.businesswire.com/news/home/20231103234375/en/
[21]CDR-LifePresentsFindingsfromTwoStudiesinPreparationofPhase1TrialwithImmunotherapyCDR404forTreatmentofSolidTumorsatSITC2023.RetrievedNovember7,2023,fromhttps://www.cdr-life.com/news_item/cdr-life-presents-findings-from-two-studies-in-preparation-of-phase-1-trial-with-immunotherapy-cdr404-for-treatment-of-solid-tumors-at-sitc-2023/
[22]BioInventPresentsPositiveDataatSITCfromClinicalPhase1/2aTrialofBI-1808asSingleAgent.RetrievedNovember7,2023,fromhttps://www.accesswire.com/799347/bioinvent-presents-positive-data-at-sitc-from-clinical-phase-12a-trial-of-bi-1808-as-single-agent
[23]SenseiBiotherapeuticsReportsFavorableClinicalDataforSNS-101at2023SITCAnnualMeeting.RetrievedNovember7,2023,fromhttps://investors.senseibio.com/news-releases/news-release-details/sensei-biotherapeutics-reports-favorable-clinical-data-sns-101
[24]HOOKIPAPharmaPresentsPositiveBiomarkerandTranslationalDataonHB-200MonotherapyatSocietyforImmunotherapyofCancer2023.RetrievedNovember7,2023,fromhttps://ir.hookipapharma.com/news-releases/news-release-details/hookipa-pharma-presents-positive-biomarker-and-translational
[25]ImCheckPresentedUpdatedPositiveDatafromPhaseI/IIaEVICTION-2TrialofICT01inCombinationwithLow-doseIL-2atSITC2023.RetrievedNovember7,2023,fromhttps://www.globenewswire.com/news-release/2023/11/03/2773393/0/en/ImCheck-Presented-Updated-Positive-Data-from-Phase-I-IIa-EVICTION-2-Trial-of-ICT01-in-Combination-with-Low-dose-IL-2-at-SITC-2023.html
[26]AutobahnTherapeuticsAnnouncesPositiveToplineResultsfromPhase1StudyofABX-002,itsLeadOralTreatmentforMajorDepressiveDisorder.RetrievedNovember7,2023,fromhttps://www.businesswire.com/news/home/20231107741595/en
[27]GateNeurosciencesAnnouncesPositiveToplineHumanEEGBiomarkerResultsDemonstratingDose-DependentTargetActivationinPhase1StudyofApimostinel.RetrievedNovember7,2023,fromhttps://www.businesswire.com/news/home/20231107912541/en
[28]ENBTherapeuticsPresentsTop-lineResultsfromPhase1bENBOLDEN-101StudyinPlatinumRefractory/ResistantOvarianCanceratSITC2023.RetrievedNovember7,2023,fromhttps://www.businesswire.com/news/home/20231107964841/en
[29]GraceScienceAnnouncesFDAClearanceofInvestigationalNewDrug(IND)ApplicationtoInitiateaPhase1/2/3TrialfortheTreatmentofNGLY1DeficiencywithGS-100,anAAV9NGLY1GeneTherapy.RetrievedNovember8,2023,fromhttps://www.businesswire.com/news/home/20231106501186/en/Grace-Science-Announces-FDA-Clearance-of-Investigational-New-Drug-IND-Application-to-Initiate-a-Phase-123-Trial-for-the-Treatment-of-NGLY1-Deficiency-with-GS-100-an-AAV9-NGLY1-Gene-Therapy
[30]InvestigatorSponsorofHCWBiologics’Phase1ClinicalTrialPresentedHumanDataReadoutandAnti-CancerMechanismofActionofHCW9218at38thSITCAnnualMeeting.RetrievedNovember8,2023,fromhttps://www.globenewswire.com/news-release/2023/11/08/2776105/0/en/Investigator-Sponsor-of-HCW-Biologics-Phase-1-Clinical-Trial-Presented-Human-Data-Readout-and-Anti-Cancer-Mechanism-of-Action-of-HCW9218-at-38th-SITC-Annual-Meeting.html
[31]ImmaticsReportsInterimClinicalDatafromACTengine®IMA203andIMA203CD8TCR-TMonotherapiesTargetingPRAMEinanOngoingPhase1Trial.RetrievedNovember8,2023,fromhttps://www.globenewswire.com/news-release/2023/11/08/2776060/0/en/Immatics-Reports-Interim-Clinical-Data-from-ACTengine-IMA203-and-IMA203CD8-TCR-T-Monotherapies-Targeting-PRAME-in-an-Ongoing-Phase-1-Trial.html
[32]ReplimunePresentsUpdatedDataonRP2inUvealMelanomaduringPlenarySessionatthe20thInternationalCongressoftheSocietyforMelanomaResearch.RetrievedNovember8,2023,fromhttps://ir.replimune.com/news-releases/news-release-details/replimune-presents-updated-data-rp2-uveal-melanoma-during
[33]SanaBiotechnologyAnnouncesFDAClearanceofInvestigationalNewDrugApplicationforSC291,aHypoimmune-modified,CD19-directedAllogeneicCARTTherapy,forPatientswithLupusNephritis,ExtrarenalLupus,andANCA-associatedVasculitis.RetrievedNovember8,2023,fromhttps://www.globenewswire.com/news-release/2023/11/09/2777840/0/en/Sana-Biotechnology-Announces-FDA-Clearance-of-Investigational-New-Drug-Application-for-SC291-a-Hypoimmune-modified-CD19-directed-Allogeneic-CAR-T-Therapy-for-Patients-with-Lupus-Ne.html
免责声明:药明康德内容团队专注介绍全球生物医药健康研究进展。本文仅作信息交流之目的,文中观点不代表药明康德立场,亦不代表药明康德支持或反对文中观点。本文也不是治疗方案推荐。如需获得治疗方案指导,请前往正规医院就诊。
