康 · 学术 | Reaction of the Day No. 1145
转自:康龙化成
Cr-CatalyzedAsymmetricCrossAza-PinacolCouplingsforβ‑AminoAlcoholSynthesis
HuiHuandZhaobinWang*
KeyLaboratoryofPreciseSynthesisofFunctionalMoleculesofZhejiangProvince,WestlakeUniversity,Hangzhou310030,China
InstituteofNaturalSciences,WestlakeInstituteforAdvancedStudy,Hangzhou310024,China
—J.Am.Chem.Soc.2023, 145, 38, 20775-20781.
RecommendedbyShiLi_MOC

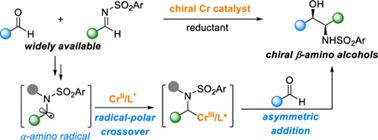
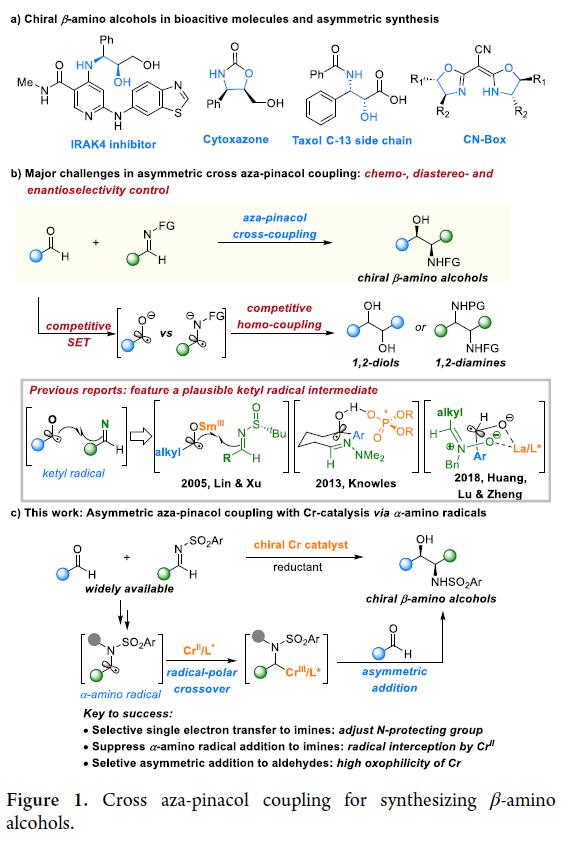
ABSTRACT:Chiralβ-aminoalcoholsarecrucialstructuralmotifsfoundinpharmaceuticals,naturalproducts,andchiralligandsinasymmetriccatalysis.Despitepreviousadvances,thedevelopmentofcatalyticapproachestoaccessβ-aminoalcoholsbearingvicinalstereocentersfromreadilyavailablechemicalsremainsaprominentchallenge.Herein,wedescribetheCr-catalyzedasymmetriccrossaza-pinacolcouplingofaldehydesandN-sulfonylimines.Thisprotocolproceedsinaradical-polarcrossovermannerfromtheintermediacyofanα-aminoradicalinsteadofaketylradical.Keytothesuccessisusingachiralchromiumcatalyst,whichplaysatripleroleinthechemoselectivesingle-electronreductionoftheimine,fastradicalinterceptiontoinhibitradicaladditiontoimines,andchemo-andstereoselectiveadditiontoaldehydesinsteadofimines.Thismethodprovidesamodularandefficientapproachtoaccessingdiverseβ-aminoalcoholsbearingvicinalstereocenters.

SelectedSubstrateScopeforAldehydes
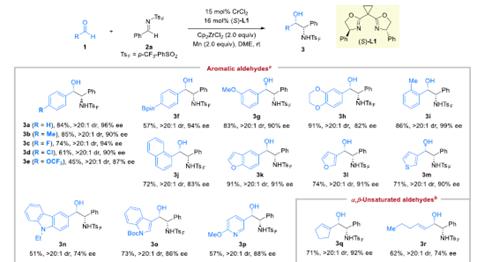
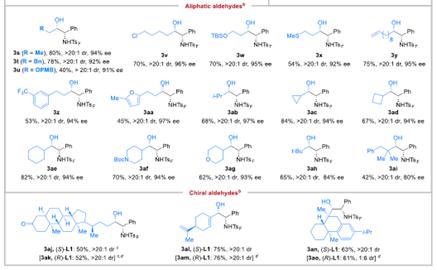
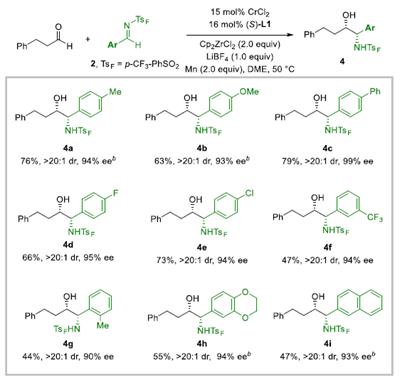
SubstrateScopeforN-SulfonylImines
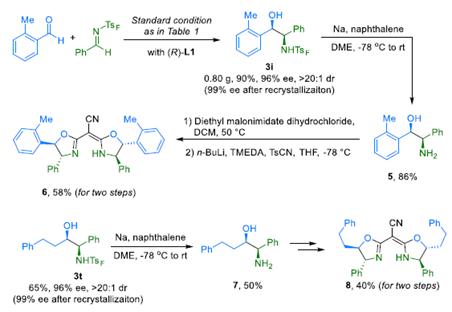
RepresentativeSyntheticApplications
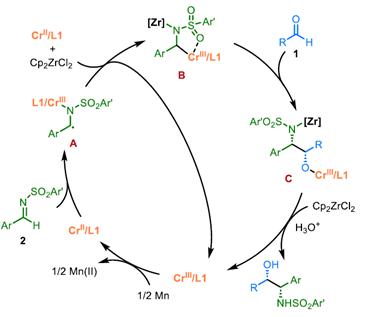
Proposed Mechanism
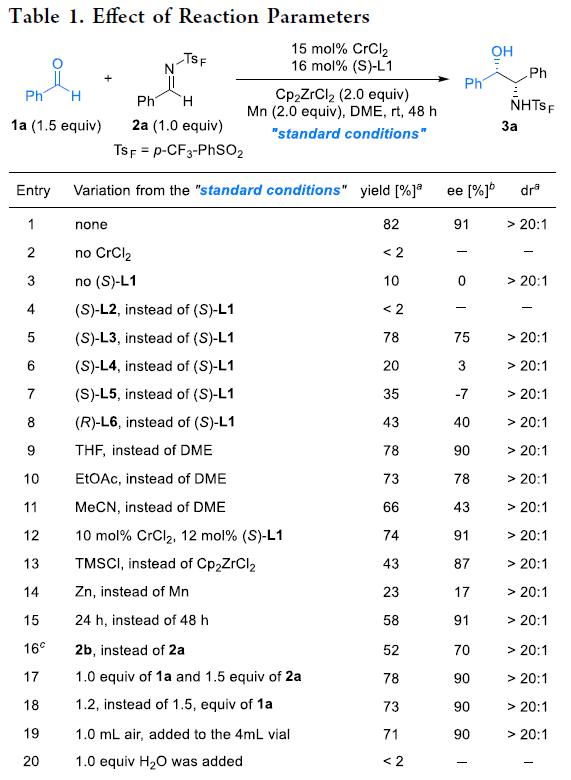
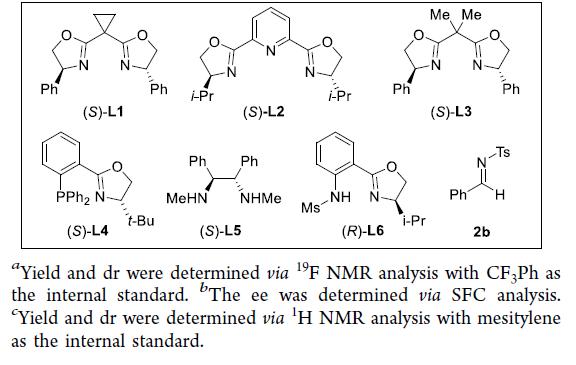
ZhaobinWang’sgroupdevelopedachromium-catalyzedasymmetriccrossaza-pinacolcouplingreactionfromreadilyavailablealdehydesandimines.Distinctfromknownstrategies,thisprotocolproceedsviatheplausibleα-aminoradicalintermediateinaradical-polarcrossovermanner.TheproperchoiceofN-sulfonylgroupiscrucialforadjustingreactivityandfacilitatingstereoselectivitycontrol.Thechiralchromiumcomplexplaysatriplecriticalroleinthechemoselectivesingle-electronreductionoftheimine,fastradicalinterceptiontoinhibitradicaladditiontoimines,andchemo-andstereoselectiveadditiontoaldehydesinsteadofimines.Theprotocolexhibitsabroadsubstratescopeandgoodfunctionalgroupcompatibility,providingmodularaccesstovariousβ-aminoalcoholsbearingvicinalstereocenters.Giventheimportanceofchiralaminoalcoholsinpharmaceuticals,agrochemicals,andasymmetricsynthesis,weanticipatethisprotocolwillfindbroadapplicationinthesyntheticcommunitytoproducefinechemicals.
ZhaobinWang小组开发了一种铬催化的醛和亚胺的不对称交叉氮杂频哪醇型偶联反应。与已知的策略不同,该反应通过形成α-氨基自由基中间体与醛的极性交叉偶联形式进行。N-磺酰基的选择对于调节反应性和立体选择性至关重要。手性铬络合物在亚胺的化学选择性单电子还原以及其自由基与醛而非亚胺的化学选择性和立体选择性加成中起着关键作用。该反应具有广泛的底物范围和良好的官能团兼容性,提供了合成各种含相邻立体中心β-氨基醇的方法。鉴于手性氨基醇在药物、农用化学品和不对称合成中的重要性,我们预计该反应将在有机合成化学中得到广泛应用。
